Cough Syrup deaths: Sresan Pharmaceuticals’ license cancelled, company shut down
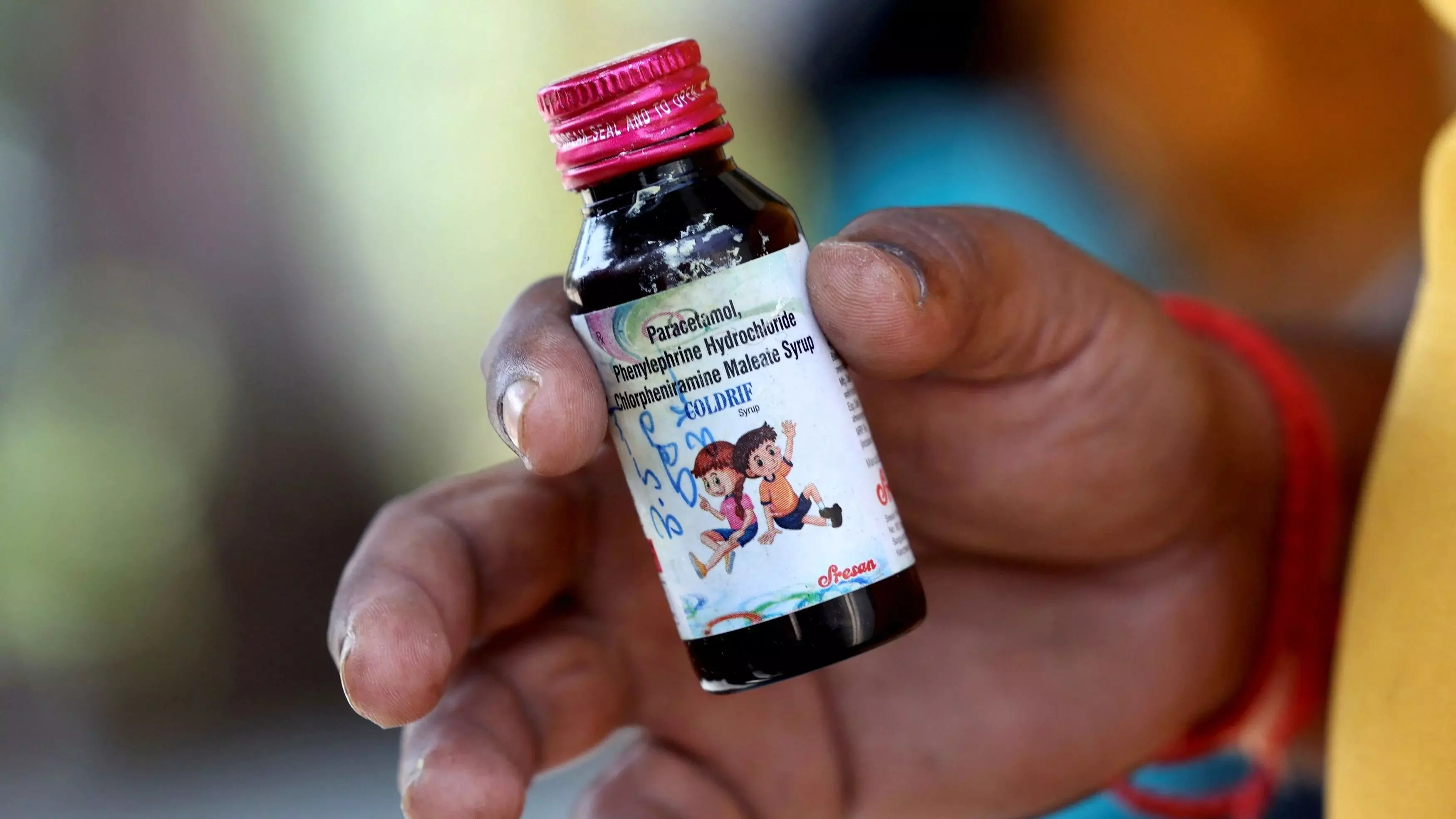
Chennai: Tamil Nadu Drugs Control Department on Monday announced the cancellation of the manufacturing license of Sresan Pharmaceutical company, the manufacturer of Coldrif cough syrup, linked with deaths of at least 22 people in Madhya Pradesh. The state health department stated that the company has been officially shut down after its investigation of toxic contaminants, specifically Diethylene Glycol (DEG), in their cough syrup, Coldrif. Additionally, the department ordered comprehensive inspections across all pharmaceutical manufacturing units in Tamil Nadu, and large-scale inspections are currently in progress throughout the State. A Parasia court sent Sresan Pharma owner Ranganathan to 10-day police custody after he was arrested on October 9 in Chennai by an SIT from Madhya Pradesh.
Two senior drug inspectors have also been suspended for dereliction of duty. The Tamil Nadu government has ordered comprehensive inspections of other pharmaceutical manufacturing companies in the state. Earlier, BJP leader K Annamalai criticised the Tamil Nadu government for its handling of a case. He highlighted that the state government suspended only two drug inspectors after the Special Investigation Team (SIT) intervened, accusing the TN government of creating an “illusion” and evading responsibility. “A drug manufactured by a private pharmaceutical company in Kanchipuram has reportedly caused the deaths of 23 people in Madhya Pradesh and three children in Rajasthan. However, the Tamil Nadu government has suspended only two drug inspectors and is trying to create an illusion that it has no connection or responsibility in this matter. Yesterday, the Drugs Controller General of India (DCGI) took an important decision. This time, the agency has decided that every medicine produced in India must undergo mandatory testing before approval,” he said. He also noted the company’s history of quality violations and the lack of inspections by Tamil Nadu drug inspectors. Additionally, the Drugs Controller General of India (DCGI) has issued a directive to all States and Union Territories, calling for strict compliance with the Drugs and Cosmetics Rules, 1945, for the testing of raw materials and finished pharmaceutical formulations.



